Research at the Stanford Stroke Center
The Stanford Stroke Center has consistently been one of the most prolific stroke research groups in the United States; faculty members have published more than 700 manuscripts based on clinical stroke research, as well as hundreds of basic science studies; the Center has maintained continuous NIH grant support for 28 years. Stroke Center faculty members have authored more than 25 national and international clinical guideline statements. Stanford has pioneered major advances in medical therapies for treating and preventing stroke, neurosurgical techniques for stroke prevention, and novel interventional neuroradiologic procedures for stroke patients. The Center developed the RAPID stroke imaging platform and designed and coordinated the three NIH-funded DEFUSE studies, which led to demonstrating the efficacy of both intravenous thrombolysis and endovascular thrombectomy in imaging-selected patients who presented late after stroke onset. The Neurocritical Care Program has made key advances in the diagnosis of intracerebral hemorrhage and the prognosis of coma. Stanford neuroscientists have helped clarify the basic mechanisms of stroke-induced brain injury and have pioneered several new imaging techniques that facilitate the identification of salvageable ischemic brain tissue in patients presenting with an acute stroke. Stroke recovery research, including participation in multiple early-phase clinical stem cell therapy trials and development of a new line of stem cells, has also been a focus area.
Research Programs
- Albers, Greg, MD
- Andreasson, Katrin, MD
- Buckwalter, Marion, MD, PhD
- Finley Caulfield, Anna, MD
- George, Paul, MD
- Hirsch, Karen, MD
- Kraler, Lironn, MD
- Lansberg, Maarten, MD, PhD
- Lee, Sarah, MD
- Mijalski, Christina, MD
- Nuyujukian, Paul, MD, PhD
- Schwartz, Neil, MD, PhD
- Steinberg, Gary MD, PhD
- Venkat, Chitra, MD
- Vora, Nirali, MD
Neurology & Neurological Sciences Stroke Research Labs
Katrin Andreasson, PhD
Professor of Neurology and Neurological Sciences
In our lab, we are interested in understanding the mechanisms by which neuroinflammation elicits synaptic and neuronal injury in chronic and acute models of neurological disease. Our foot in the door has been the study of the cyclooxygenase-2 (COX-2) pathway and its downstream prostaglandin receptor signaling pathways, which function in important ways in modulating the inflammatory response in brain in models of Alzheimer's disease (AD), amyotrophic lateral sclerosis (ALS), Parkinson's disease (PD), and stroke. Thus this pathway functions across a broad spectrum of neurodegenerative diseases, and may potentially modulate inflammatory responses and neuronal injury via conserved cellular and molecular mechanisms. We use genetic and pharmacologic strategies as well as in vitro culture approaches to define COX-2/prostaglandin receptor mediated mechanisms of action in eliciting synaptic and neuronal injury in models of human neurological disease. Our long-term goal is to (1) further understand how neuroinflammatory processes injure synapses and neurons and disrupt circuits, (2) define the contribution of the COX-2/prostaglandin signaling pathways in this process, and (3) develop therapeutic strategies targeting injurious inflammatory processes in human neurological diseases.
Marion Buckwalter, MD, PhD
Professor of Neurology and Neurological Sciences, and Neurosurgery
Our lab focuses on how inflammatory responses after brain injury affect neurological recovery. In the United States, there are 4 million people currently living with the effects of stroke, and another 4.3 million living with the effects of traumatic brain injury. Of the people who have had a stroke, many are disabled to the degree that they cannot work, and a significant proportion are unable to walk, feed themselves, or communicate with their families the way they could prior to their stroke. Despite this very high number of people who are suffering, there is a large knowledge gap regarding the mechanisms by which neurological recovery occurs, and not a single FDA-approved therapy available to help people recover. There is reason to think that such a therapy might be obtainable – we know that some people, especially younger ones, experience significant recovery after stroke. Animal studies, almost entirely done in young animals, also demonstrate significant recovery after neurological injury. Our goal is thus to better understand the mechanisms that contribute to recovery in the young, and how they are influenced by inflammatory responses. Once we understand this, we hope to be able to develop new therapies to help people’s brains repair themselves.
Paul George, MD, PhD
Assistant Professor of Neurology and Neurological Sciences, and Neurosurgery
The George lab applies bioengineering approaches to explore neurological disorders. Our particular focus is utilizing interactive biomaterials to promote neural recovery. Through the use of biomaterials, microfabrication techniques, and stem cell therapeutics, we are able to manipulate the neural environment and determine important pathways for healing. Our goal is to use these pathways to develop new treatments for patients with stroke and other neurological diseases.
With nearly 800,000 strokes occurring annually in the United States alone, stroke remains a leading cause of long term disability and death in the world. Despite stroke’s prevalence, currently there are no medical therapies to improve subacute and chronic stroke recovery. The George lab strives to increase our understanding of naturally occurring repair mechanisms through biomarkers and novel technologies to improve the care of stroke survivors.
Neurosurgery Stroke Research Labs
Gary K. Steinberg, MD, PhD
Co-Director, Stanford Stroke Center
Director, Stanford Institute for Neuro-Innnovation and Translational Neurosciences
Bernard and Ronni Lacroute-William Randolph Hearst Professor of Neurosurgery and the Neurosciences
Our laboratory is interested in elucidating the mechanisms of brain repair and recovery after stroke with the long term goal of finding novel therapeutic strategies to promote stroke recovery. Brain plasticity and remapping is a key repair process after stroke and we study this at the circuit level using optogenetics, and at the synaptic level using electrophysiology and array tomography. A major focus of our work is to understand how transplanted neural stem cells modulate this brain plasticity, and other repair pathways related to angiogenesis and inflammation, using genetic mouse models, gene profiling, and gene transfer techniques. Identifying the molecular mechanisms of stem cell-mediated brain recovery after stroke will enable us to manipulate the system to optimize stem cell efficacy, and could also lead to the identification of novel drug targets for stroke.
Our clinical research efforts focus on novel approaches for treating intracranial aneurysms, intracranial and spinal vascular malformations, occlusive cerebrovascular disease such as Moyamoya disease and stroke. These include advances in microsurgery, interventional neuroradiology, stereotactic radiosurgery, 3D imaging, surgical navigation, revascularization techniques, the use of mild brain hypothermia and other clinical neuroprotective agents, and neurotransplantation.
Paul Nuyujukian, MD, PhD
Assistant Professor of Bioengineering and of Neurosurgery
The Brain Interfacing Laboratory is interested in the applicability of brain-machine interfaces as a platform technology for a variety of brain-related medical conditions, particularly stroke and epilepsy. This research spans both preclinical models and human clinical studies.
With respect to stroke, we are exploring how the brain recovers from injury in translational preclinical models. This investigation leverages multichannel electrode arrays to gather a neural population estimate of the state of the brain. The goal here is to develop and then leverage a neural biomarker to guide stroke rehabilitation, with the aim of improving both the rate and total recovery from stroke.
The Stanford Stroke Center is recognized as a world-leader in clinical stroke research. Through collaborations between Stroke Neurology, Interventional Neuroradiology, Neurosurgery and Engineering the Stanford Stroke Center continuously seeks to develop and test new methods to optimize the treatment of stroke patients. The Stanford Stroke Center has developed new ways to image the brain of patients suffering a stroke. This research has made it possible to individualize stroke treatment and expand the number of patients who can undergo highly effective treatments for their stroke. Our research team has also pioneered the development and testing of stem cell treatments for stroke recovery, a yet unproven but promising new therapy to restore function after stroke. We have developed new surgical techniques to treat stroke, discovered better ways to predict the outcome of patients who have suffered neurological injury after cardiac arrest, and been part of numerous clinical trials aimed at establishing the best way to prevent stroke recurrence in our patients. More details about some of our clinical research projects are described below.
The basic premise underlying acute stroke therapy is to salvage the ischemic region from evolving into infarction, thereby maintaining brain function and improving outcome. The concept of the ischemic penumbra concept envisions not only potentially salvageable or at-risk ischemic tissue but also nonviable tissue known as the “ischemic core”. The Stanford Stroke Center has been at the forefront of developing acute imaging and image processing techniques that provide immediate and accurate visualization of both core and penumbra. These techniques, which involve MRI with diffusion weighted imaging (DWI) and perfusion weighed imaging (PWI), have proven to identify patients who can benefit from both intravenous and intra-arterial therapies well beyond established time frames.
A research collaboration coordinated at Stanford helped establish that a lesion detected by DWI lesion is an extremely accurate surrogate for the ischemic core. Subsequently, this concept was translated to CT perfusion imaging with thresholded relative CBF maps.
Data from Stanford demonstrated that perfusion imaging, when optimally processed, can accurately identify critically hypoperfused penumbral tissue.
The DEFUSE Study
This Stanford study, sponsored by the NIH, demonstrated that patients with a favorable MRI profile, called Target Mismatch, have excellent outcomes following reperfusion, even when treated up to 6 hours after symptom onset with iv tPA. Other MRI profiles, including the No mismatch and Malignant profile failed to show any evidence of benefit from reperfusion.
RAPID Software
In order to automatically process advance stroke imaging data quickly and accurately, Stanford Stroke Center faculty members developed a unique software platform called RAPID. Using the databases from multiple international studies, this software program has been demonstrated to identify patients who benefit from reperfusion following late window iv tPA therapy and subsequently endovascular therapy.
DEFUSE 2
This multicenter trial was designed and run by Stanford and funded by the NIH. The trial results demonstrated that using the RAPID software, selected patients can be identified who benefit for intra-arterial clot removal therapy up to 12 hours after symptom onset. These research findings demonstrated that the paradigm for acute stroke treatment could move away from arbitrary time windows. Identification of salvageable brain tissue and determining the site of vascular obstruction has become the focus of acute imaging. DEFUSE 3 and DAWN then demonstrated that treatment strategies can then be individualized based on imaging findings and leading to dramatic clinical benefits even in late treatment windows.
DEFUSE 3
DEFUSE 3 was a 38-center NIH-funded study led by the Stanford Stroke Center that demonstrated that nearly half of all patients treated between six and 16 hours after the onset of their symptoms could be largely spared from the consequences of their stroke and the number of stroke patients who died or required confinement to nursing homes was nearly cut in half. These results represent the largest improvements seen in any stroke-related trial to date. This clinical trial demonstrated that far more people than previously thought can benefit from thrombectomy for acute ischemic stroke.
The improved outcomes were achieved through the use of the RAPID software platform for patient selection. This software helps identify stroke patients who continue to have salvageable brain tissue long after the therapeutic window had generally been considered helpful has closed. Results of the trial were published in The New England Journal of Medicine and coincided with AHA new acute-stroke treatment guidelines that expanded the stoke treatment window from 6 to 24 hours.
Stanford Neurologists Play Key Role In Redefinition Of TIA, Determining Prognosis And Optimal Management
In a 2002 article in the New England Journal of Medicine, Stanford Neurologist Greg Albers, MD, and other cerebrovascular experts called for a revision in the definition of TIA — from time-based (the resolution of symptoms in 24 hours) — to the presence or absence of brain infarction, a tissue-based definition, on neuroimaging. In 2009, the American Stroke Association released a guideline endorsing this change in the definition of TIA.
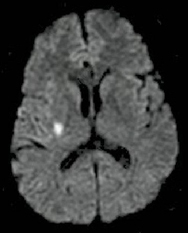 |
Image shows a DWI positive lesion in a patient with transient left sided weakness. |
The benefits of the new TIA definition have been demonstrated in a series of recent publications in Lancet Neurology, Stroke, and Neurology that were co-authored by Stanford Stroke Center neurologists. These studies have documented that that identifying a small area of brain injury on the MRI is much more powerful than clinical data alone to predicting early stroke risk; in fact early stroke risk is about 15 times higher in patients with TIA symptoms who have a small area of tissue injury on the MRI compared to those who have a normal MRI.
High risk patients should be admitted to the hospital for close observation so that tPA can be administered rapidly if a stoke occurs, and to facilitate urgent completion of a full diagnostic evaluation. Low risk TIA patients can be managed safely and cost-effectively in an outpatient TIA clinic. A recent Stanford study, TWO ACES, documented that this novel TIA strategy resulted in extremely low stroke rates and high patient satisfaction.
Images demonstrate a PWI positive lesion (arrows) in a patient with transient right sided weakness/numbness.
The Stanford TIA program is currently evaluating a new technology, perfusion weighted imaging (PWI), as a method of confirming an ischemic “footprint” that can verify a transient neurological episode was caused by ischemia, rather than a non-ischemic condition. In a preliminary study, Stanford Stroke Center neurologists demonstrated that about 30% of patients with symptoms suggestive of a TIA have a positive PWI scan.
Stanford has collaborated with the National Stroke Association, the American Heart Association and the American College of Chest Physicians to produce several guideline statements aimed at refining the diagnosis and management of TIA:
National Stroke Association Recommendations for TIA
Guidelines for the Prevention of Stroke in Patients with TIA
Definition and Evaluation of Transient Ischemic Attack
Antithrombotic and thrombolytic therapy for ischemic stroke
The CTP to predict Response to recanalization in Ischemic Stroke Project (CRISP) is a study funded by the National Institutes of Health (NIH) to develop a practical tool to identify acute stroke patients who are likely to benefit from endovascular therapy.
Stroke is the number one cause of disability in the United States. It is caused by an occlusion of a blood vessel in the brain. In order to reduce the burden of disability caused by stroke there is a need for better stroke treatments that are available to more stroke victims. Endovascular stroke treatment is increasingly being used and may fill this need, as it can be very effective at opening up occluded blood vessels in the brain. It is, however, not known which patients benefit clinically.
Previous studies suggest that stroke patients with a small volume of irreversible ischemic injury (infarct core) and a large volume of reversible ischemic injury (penumbra) are most likely to benefit from restoration of blood flow. MRI shows promise for identification of the ischemic core and penumbra but it has very limited availability in US emergency rooms. Computed Tomography Perfusion (CTP) imaging is a potential solution as it is widely available and can easily be added to a non-contrast head CT, already routinely obtained to evaluate stroke patients in the emergency room. However, methods for processing of CTP images and criteria for interpretation of the images are still immature. Therefore, patient selection based on CTP images is not ready for implementation in clinical trials or clinical practice.
As part of the CRISP study we have developed a fully automated system (RAPID) for processing of CT Perfusion (CTP) images that generates brain maps of the ischemic core and penumbra. We have defined criteria, based on these CTP maps, which we believe will predict if a patient is likely to benefit from restoration of blood flow. We are currently conducting a prospective cohort study at Stanford University and several collaborating hospitals across the USA to test if physicians in the emergency setting, with the aid of RAPID, can accurately predict if a patient will benefit from an endovascular revascularization procedure.
The successful execution of this research will provide physicians with an easy, automated method to select patients who are likely to benefit from restoration of blood flow. Such a method, thanks to easy accessibility to CT technology, would be of great value for patient selection in multi-center clinical acute stroke trials and, eventually, in routine clinical practice.
The looming healthcare financial crisis in America led to the creation of the Stanford Clinical Excellence Research Center (CERC). The mission of the program is: "better health, less spending." CERC brings together individuals with backgrounds in medicine, industrial engineering, and management and social sciences in an effort to create innovative healthcare delivery models that safely lower per capita spending while maintaining or improving health outcomes and patient experience. The goal is to bend the national trend of the ever increasing portion of our national GDP being spent on healthcare. Stanford Stoke Center faculty member Amy Tai is collaborating with CERC on a novel stroke/TIA heath care delivery project.
Stroke is the leading cause of disability and eighth most expensive health condition in America. Dr. Tai spent a year working on a redesigned care delivery process to address the gaps in the current system of stroke care in the United States. An innovative stroke care model was developed using a systematic design method that included: (1) A literature review of major guidelines, stroke care delivery methods, and cost effectiveness studies to identify the best practices and how to achieve them at the lowest cost; (2) Site visits with those identified to be best at delivering high quality care for the lowest cost; (3) Observations of patients and providers to identify their unmet needs; (4) Development of a model to address stakeholders' unmet needs using methods from design thinking and health care delivery science; (5) Estimation of the model's cost-saving potential, using national averages for risk factor prevalence, stroke incidence, and costs of conditions and interventions.
Synthesis of this design process yielded a high-value stroke care model that: (1) prevents strokes through maximal use of preventative medications; (2) stratifies care for patients with TIA and mild stroke symptoms and; (3) delivers tPA in most time-efficient way possible and delivers a strengthened transition to community program for those at high risk for readmissions. It is estimated that this model will significantly improve patient outcomes and reduce healthcare spending in cerebrovascular disease by 11%.
Intracerebral hemorrhage (ICH) is a devastating type of stroke caused due to bleeding within the brain tissue. It affects about a million patients worldwide every year and has the highest mortality and morbidity of any type of stroke. Of all patients who present with a stroke 10-20% will have suffered a spontaneous (non-traumatic) ICH rather than an ischemic stroke. This percentage is higher among Black, Asian, and Hispanic populations, and expected to rise in the United States over the next few decades, due to increasing age and continuing changes in racial demographics.
ICH occurs due to a variety of causes including hypertension, cerebral amyloid angiopathy, excessive anticoagulation, vascular malformations, cerebral venous thrombosis or brain tumors. The acute treatment, prognosis and prevention of recurrent ICH depend on the reason for the brain hemorrhage. For example, a patient who has an ICH due to cerebral amyloid angiopathy will need to avoid blood thinners to decrease the probability of a recurrent ICH. On the other hand, a patient with ICH due to cerebral venous thrombosis will require blood thinners for treatment.
ICH is readily diagnosed by CT, which is typically the first imaging test performed during the initial diagnostic evaluation. CT provides information on the size and the location of the hematoma. However, although CT is very sensitive for the detecting of acute blood in the brain, it often does not provide information that allows determination of the cause of the hemorrhage. While magnetic resonance imaging (MRI) has substantially improved our diagnostic capabilities, the appropriate use of MRI and its effectiveness has not been studied systematically in these patients. Furthermore, it is unclear whether routine MRI in ICH yields clinically relevant data and if this data will change management decisions regarding further diagnostic testing and therapeutic options above and beyond that which can be achieved by CT and cerebral angiography. These questions have major ramifications for the care of patients with ICH or IVH. If MRI truly can categorize patients into specific diagnostic categories better than CT, this would represent a major paradigm shift in the way that these patients are typically evaluated. On the other hand, because of the added expense of MRI, its general use could result in a substantial increase in the cost of neurological care. These added costs must result in improvements in patient management in order to justify the added financial resources involved.
DASH (Diagnostic accuracy of MRI in Spontaneous Intracerebral Hemorrhage) is a prospective study funded by the National Institute of Health (NIH). The overall aim of this project is to prospectively determine whether MRI can improve the conventional neuroradiological evaluation of patients with a spontaneous ICH or IVH. The study design will also allow us to identify the added benefit of specific MR sequences (including novel state-of-the-art sequences) and repeat MRI in the chronic stage, thereby allowing us to prospectively determine their value in a consecutive series of patients. This information should have a major impact on the management of these patients by providing data on the diagnostic yield of routine MRI in patients presenting with a wide variety of causes for ICH or IVH. These data will help guide the diagnostic evaluation and the management of brain hemorrhage patients in the future. In addition, the data derived from this study will make a substantial contribution to future patient management by facilitation of the development of evidence based practice guidelines for the use of MRI in the workup of patients presenting with spontaneous ICH or IVH.
Publications
- Natural History and Prognostic Value of Corticospinal Tract Wallerian Degeneration in Intracerebral Hemorrhage. Venkatasubramanian C, MD, Jonathan T. Kleinman MD, Nancy J. Fischbein MD, Jean-Marc Olivot MD, PhD, Alisa D. Gean MD, Irina Eyngorn MD, Ryan W. Snider BA, Michael Mlynash MD, MS, and Christine A.C. Wijman MD, PhD. J Am Heart Assoc. 2013
- Magnetic resonance imaging profile of blood-brain barrier injury in patients with acute intracerebral hemorrhage. Aksoy D, Bammer R, Mlynash M, Venkatasubramanian C, Eyngorn I, Snider RW, Gupta SN, Narayana R, Fischbein N, Wijman CA. J Am Heart Assoc. 2013; 2 (3): e000161
- Natural history of perihematomal edema after intracerebral hemorrhage measured by serial magnetic resonance imaging. Venkatasubramanian C, Mlynash M, Finley-Caulfield A, Eyngorn I, Kalimuthu R, Snider RW, Wijman CA. Stroke. 2011; 42 (1): 73-80
- MRI profile of the perihematomal region in acute intracerebral hemorrhage. Olivot JM, Mlynash M, Kleinman JT, Straka M, Venkatasubramanian C, Bammer R, Moseley ME, Albers GW, Wijman CA. Stroke. 2010; 41 (11): 2681-3.
- Utility of early MRI in the diagnosis and management of acute spontaneous intracerebral hemorrhage. Wijman CA, Venkatasubramanian C, Bruins S, Fischbein N, Schwartz N. Cerebrovasc Dis. 2010; 30 (5): 456-63
PRECISE - Perfusion imaging to identify posterior circulation candidates for thrombectomy
The purpose of this research is to find out if advanced brain imaging can help identify which patients who have suffered an ischemic stroke (a blockage of blood flow) to the back of their brain are most likely to benefit from a procedure to remove the blood clot to restore the blood flow to the brain.
Protocol ID: Pro00053806
PI: Gregory Albers, MD
Study Coordinator: Irina Eyngorn
Status: RECRUITING
CRISP 2 - CT Perfusion to Predict Response to Recanalization in Ischemic Stroke Project
The purpose of this research is to analyze brain imaging data and clinical data to understand how stroke progresses through the brain.
Protocol ID: 56227
PI: Maarten Lansberg, MD, PhD
Study coordinator: Leonel Lugo
Status: RECRUITING
SATURN -Statins Use in Intracerebral Hemorrhage Patients
This research is being done to find out if it is better to continue or discontinue statin drugs (a type of cholesterol-lowering medication) in people who had a brain hemorrhage (bleeding in the brain) while taking such a statin drug. It will also examine if having certain genes (apolipoproteine E) influences the likelihood of getting a brain hemorrhage while taking a statin drug.
Protocol ID: 55536
PI: Chitra Venkatasubramanian, MD
Study coordinator: Madelleine Garcia
Status: RECRUITING
ASPIRE: Anticoagulation in ICH (intracerebral hemorrhage) Survivors for Stroke Prevention and Recovery
The purpose of this study is to compare the effects of a drug called apixaban with aspirin in patients with atrial fibrillation (irregular heart beating) and a recent brain hemorrhage (bleeding in the brain) to see which is better in preventing strokes and death.
NCT03907046
IRB #: 52983
PI: Chitra Venkatasubramanian, MD
Study coordinator: Madelleine Garcia
Status: RECRUITING
BEACH: Biomarker and Edema Attenuation in IntraCerebral Hemorrhage (BEACH)
The purpose of this research study is to find out if a study drug called MW189 is safe and well tolerated in patients with bleeding in the brain (Intracranial Hemorrhage or ICH), and whether it has a beneficial effect on patients’ outcomes and recovery. MW189 is an anti-inflammatory drug candidate being studied as a possible treatment to reduce the excessive inflammation and brain swelling.
NCT05020535
IRB #: 295926
PI: Chitra Venkatasubramanian, MD
Study coordinator: Madelleine Garcia
Status: RECRUITING
Librexia Stroke Study: Efficacy and Safety of Milvexian, an Oral Factor XIa Inhibitor, for Stroke Prevention after an Acute Ischemic Stroke or High-Risk Transient Ischemic Attack
The purpose of this research study is to see if an experimental drug, called milvexian in addition to standard of care, is safe and useful in reducing the risk of future ischemic stroke in participants after ischemic stroke or transient ischemic attack compared to placebo (tablet with no active drug) in addition to standard of care.
IRB #: 70999
PI: Nirali Vora, MD
Study coordinator: Leonel Lugo
Status: RECRUITING
CIRCA Registry: The CIRCA Chronotype and Stroke Registry: A Multicenter Study of the Association of Chronotype with Presentation, Course, and Outcome of Acute Cerebral Ischemia
The goal of this study is to figure out how natural sleep patterns, known as "chronotype," affect the course of stroke and stroke treatment.
IRB #: 72264
PI: Gregory Albers, MD
Study Coordinator: Frances Lizbeth Palomata, MBA
Status: RECRUITING
Home BP Cuff Study: Improving Blood Pressure Control in Stroke Patients by Increasing Access to a Home Blood Pressure Monitor
The purpose of this study is to investigate if providing focused teaching and a free blood pressure cuff to patients at the time of discharge from the hospital helps to facilitate improved blood pressure control. Eligible patients are those who have high blood pressure and are deemed to be at risk for stroke.
Protocol ID: 68463
PI: Lironn Kraler, MD
Study coordinator: Madelleine Garcia
Status: RECRUITING
Sodium imaging Study: Ischemic Brain Damage & Triple/Single Quantum Sodium MRI
The objective of this study is to use a new method of Magnetic Resonance Imaging (MRI) to identify the sodium concentration in brain tissue in patients with a stroke. We hope that this new method may be helpful in assessing the outcomes of potential therapies for acute stroke.
IRB #: 64720
PI: Fernando Boada, PhD
Study coordinator: Irina Eyngorn
Status: RECRUITING
StrokeCog
The purpose of this research study is to understand the long-term effects of stroke on a person’s memory and thinking. To determine this, we will schedule stroke patients to come in on a yearly basis for memory testing and collection of a small amount of blood.
Protocol ID: 42089
PI: Maarten Lansberg, MD, PhD
STATUS: RECRUITING