Home > Researcher Resources > Clinical Research Unit (CRU)
Clinical Research Unit (CRU)
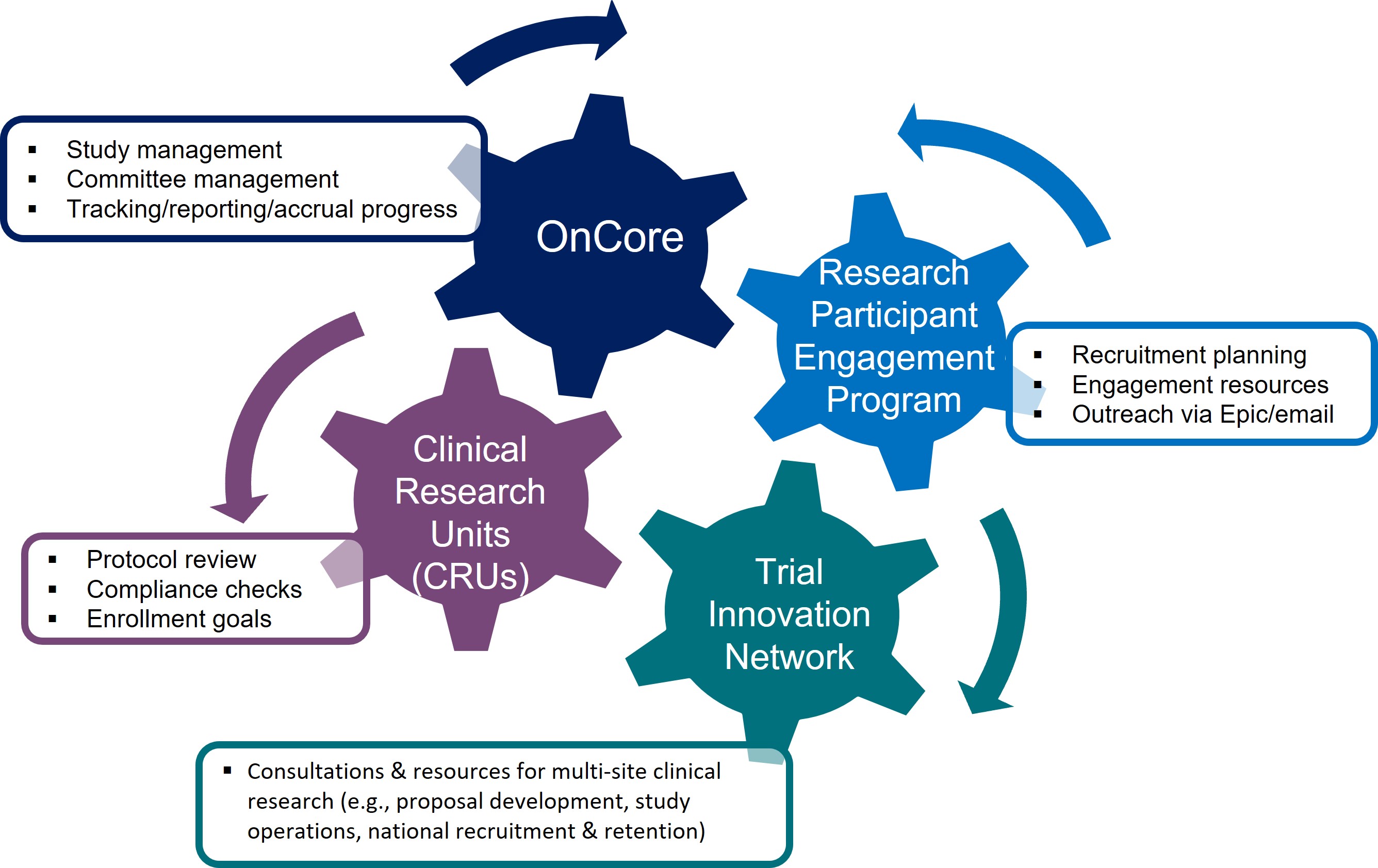
The CRUs, as a component of Stanford's CTSA hub, are charged with the continuous development and enhancement of a robust, well-organized and productive clinical and translational research program that can effectively leverage the growing clinical enterprise. This program is focused on facilitating an organizational framework at the department/division level to enhance the efficiency and quality of clinical research conducted at Stanford.