August 24, 2011 - By Krista Conger

Irving Weissman
Tissue-specific adult stem cells are responsible for the ability of mammals to re-grow the tips of fingers or toes lost to trauma or surgery, say researchers at the Stanford University School of Medicine. The finding discredits a popular theory that holds that previously specialized cells regress, or dedifferentiate, in response to injury to form a pluripotent repair structure called a blastema.
“We’ve shown conclusively that what was thought to be a blastema is instead simply resident stem cells that are already committed to become specific tissue types,” said Irving Weissman, MD, director of Stanford’s Institute for Stem Cell Biology and Regenerative Medicine. “The controversy about limb regeneration in mammals should be over.”
The research is particularly meaningful because some scientists and national media reports have championed the idea that money allotted by the California Institute for Regenerative Medicine for stem cell studies would have been better funneled to blastema research, Weissman said.
Weissman, who is also the Virginia & D.K. Ludwig Professor for Clinical Investigation in Cancer Research and a member of Stanford’s Cancer Institute, is the senior author of the study, published Aug. 24 in Nature. Postdoctoral scholar Yuval Rinkevich, PhD, is the first author.
Although it’s not well-known, mice and even some humans can re-grow finger or toe tips that have been lost in accidents. But, unlike salamanders or newts, their ability is limited to the repair of relatively minor damage. “While lower vertebrates can regenerate an entire limb within a matter of weeks, mice and humans have maintained only a vestige of this ability,” said Rinkevich. “The re-growth of amputated digit tips — a few millimeters in mice and up to the first joint in humans — is the only documented case of limb regeneration in mammals. We wanted to understand the basic mechanism of how this happens.”
Unlike salamanders, mice offered a genetically well-documented animal model with which to study limb regeneration. Specifically, Rinkevich, Weissman and their colleagues have shown that damage to a digit tip is repaired by specialized adult stem cells that spend their lives quietly nestled in each tissue type. Like master craftsmen, these cells spring into action at the first sign of damage, working independently yet side-by-side to regenerate bone, skin, tendon, vessels and nerves. But just as you wouldn’t ask a mason to wire your house, or an electrician to put on a new roof, the division of labor among these stem cells is strict. Each is responsible solely for its own tissue type.
In contrast, the blastema theory invokes a new pluripotent cell type formed out of urgency from previously specialized cells. This jack-of-all-trades cell discards its former profession and instead jumps in to indiscriminately regenerate all the tissue types of the limb.
A German group headed by stem cell scientist Elly Tanaka, PhD, published similar results in salamanders in 2009, but it was unclear whether the findings would hold true in mammals.
“This finding changes the current dogma of limb regeneration, from pluripotent blastema cells to tissue-specific stem and progenitor cells,” said Rinkevich.
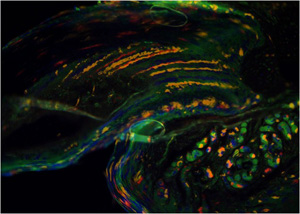
Limb regeneration is governed by the action of tissue-specific adult stem cells. Here, a mouse has been engineered to have cells that express only one of four possible colors: red, green, yellow or blue. As the tip of an amputated digit re-grows, strict separations between the contributions of individual cells are apparent as distinct bands of color.
For this study, Rinkevich used genetics to label specific tissue types in the mice with distinctive fluorescent colors that are easily seen through a microscope. He then surgically removed a small (a few millimeters) portion of the animals’ toe to mimic a naturally occurring amputation injury and waited for the tip to re-grow. (The animals were anesthetized during the procedure and were given analgesics after the procedure to relieve pain.)
After three months, Rinkevich and his colleagues examined the regenerated tissue.
“We found that each tissue type could only give rise to that type of tissue,” he said. “There was no cross contribution between tissue types or germ layers.” In other words, there were clear demarcations between areas of color that corresponded to structures such as the epidermis, tendon, nail, vessels, nerves and bone.
“I was extremely surprised,” said Rinkevich. “I began the experiment very eager to find something like a dedifferentiation or transdifferentiation phenomenon — that is, one tissue type becoming another. But this is clearly not the case.”
In addition to the blastema theory, there was one other possibility. Some researchers had suggested that stem cells circulating in the blood could contribute to this type of regeneration. To assess this possibility, Rinkevich connected the circulatory systems of two mice. One mouse was genetically bred to express a colorful marker in all its cells; the other had its toe tip removed. They found that the labeled cells did not contribute to the regenerated tissue, showing that circulating stem cells were unlikely to be involved in the regrowth of the limb.
The researchers work doesn’t discredit previous work by researchers at Stanford and elsewhere showing that it is possible using transferred genes to coax adult, specialized cells (such as those found in the skin) to become other types of cells, such as neurons. That line of study is still very important to the possible development of future therapies and the generation of cell lines for research, they say. However, the approach in the new study has some particular advantages.
“Here we are characterizing and learning about a naturally occurring regeneration phenomenon without adding other genes,” said Rinkevich. “That’s our strong point. We want to first understand how normal tissue regeneration works. Then we can try to exploit that knowledge to perhaps enhance the growth of digits or limbs in humans.”
In addition to Rinkevich and Weissman, other Stanford researchers involved in the study include research assistant Paul Lindau, postdoctoral scholar Hiroo Ueno, PhD, and professor of surgery Michael Longaker, MD.
The research was supported by the California Institute of Regenerative Medicine, the Smith Family Trust, the Oak Foundation, the Hagey Laboratory for Pediatric Regenerative Medicine, the Human Frontier Science Program and the Machiah Foundation.
About Stanford Medicine
Stanford Medicine is an integrated academic health system comprising the Stanford School of Medicine and adult and pediatric health care delivery systems. Together, they harness the full potential of biomedicine through collaborative research, education and clinical care for patients. For more information, please visit med.stanford.edu.