October 4, 2006 - By Krista Conger
Award honors work in RNA interference – gene silencing by double-stranded RNA
STANFORD, Calif. — The Nobel Assembly at Karolinska Institutet today awarded the Nobel Prize in Physiology or Medicine for 2006 to Andrew Fire, PhD, of Stanford University School of Medicine, and Craig C. Mello, PhD, of the University of Massachusetts Medical School, for their discoveries related to RNA interference.
"I was very surprised," said Fire, professor of pathology and of genetics, of the early morning phone call from the committee. "At first I thought that maybe they had a wrong number, or that I was dreaming. But I guess it's real."
Fire, 47, and Mello, 45, are part of a team of researchers credited with recognizing that certain RNA molecules can be used to turn off specific genes in animal cells. The discovery, made while Fire was at the Carnegie Institution's Department of Embryology in Baltimore, marked the first time that biologists were able to selectively "silence" the voice of one gene in the cacophony of the tens of thousands that give a cell its marching orders from development to death. Their description of the process, called RNA interference or RNAi, in Nature in 1998, jumpstarted a new biological field by opening up previously inaccessible areas of research.
It has also shown remarkable clinical promise. RNAi-based treatments are being tested in many animal models of disease — high cholesterol, HIV, cancer and hepatitis, among others — and clinical trials have been launched in humans with specific types of macular degeneration and pneumonia.

Andrew Fire, PhD
"This is an extraordinary achievement for Andy Fire and Craig Mello, for science and for Stanford," said Philip Pizzo, MD, dean of the Stanford School of Medicine. "It serves as an affirmation of the importance of basic fundamental research that yields new insights into important biological mechanisms. Such discoveries not only elucidate new understanding of human biology, but can unfold into new directions that can potentially translate into discoveries of new diagnostic and therapeutic approaches for a variety of human disorders."
Many high school biology students are familiar with the central tenet of genetics, which holds that double-stranded DNA makes single-stranded messenger RNA that makes protein, which is the workhorse of the cell. There is also double-stranded RNA, which was thought to only occur in some viruses and in a kind of nomadic gene called a transposon-both potentially deadly. As a defense, plant and animal cells developed a way to not only seek out and destroy double-stranded RNA, but also any matching messenger RNA, short-circuiting the invading gene's path to protein.
We now know that this tactic worked so well that cells adopted double-stranded RNA as way to toggle their own genes on and off like light switches. The process is catalytic, meaning that even a tiny amount of double-stranded RNA can trigger a wave of destruction.
Despite some intriguing hints that RNA was more than just an assembly manual for proteins, much of this process remained a mystery until the publication of Fire and Mello’s findings in 1998. In a series of elegant experiments, Fire, Mello and their colleagues showed that they could selectively silence an individual gene in roundworms by injecting a double-stranded version of its messenger RNA. This “RNA silencing” has since been shown to work in nearly every animal cell except yeast. (Fire and Mello’s co-authors on the 1998 paper include SiQun Xu, Mary Montgomery, Stephen Kostas and Sam Driver.)
“The beauty of this is that the work essentially happened in real time,” said David C. Schwartz, PhD, the Kellett Professor of Genetics and Chemistry at the University of Wisconsin-Madison, and a longtime friend of Fire’s. “The discovery and characterization of these RNAs is fairly recent, it’s remarkably fast. It’s just gorgeous work that stands a chance to really change medicine, aside from a remarkable tool for biology.”
Colleagues often describe Fire as remarkably modest in a field that has at least a few large egos, and on the morning Fire received news of his winning the Nobel, he lived up to that reputation. He made sure to credit “insightful and dedicated colleagues and students” with whom he has worked and “whose ideas and efforts are very much the subject of the prize.” And he noted that scientists also share a responsibility to society at large. “All of us in science look forward to sharing with the public both the responsibilities and opportunities that arise as we understand more about the human body,” he said.
He added, "For me personally, the occasion of such an award is an opportunity to thank the many patient teachers and mentors who have opened doors to science and research, and especially my family, who have made everything possible.
"This day is a wonderful chance to acknowledge that science is a group effort," Fire continued. "The advances cited in the Nobel award grew from original scientific inquiry from numerous research groups throughout the world." He thanked the National Institute of General Medical Sciences for providing the grants that made the research possible and continues to support both scientists.
“This honor underscores the fundamental role that basic research plays in advancing our understanding of health,” said Jeremy M. Berg, PhD, director of the National Institute of General Medical Sciences. “The unanticipated discovery of a basic biological process that can silence genes took the biomedical research community by storm. RNAi is both a powerful tool for studying gene function and a promising approach to treating a host of human diseases, from macular degeneration and cancer to flu and other infections.”
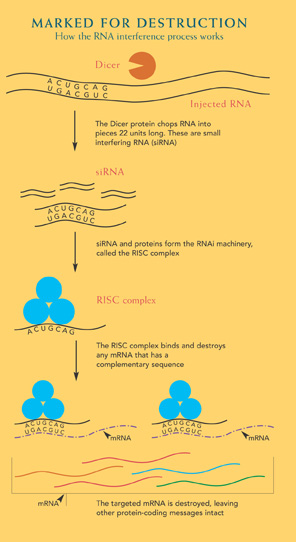
Like Fire, double-stranded RNA doesn’t act alone. It teams up, first with an aptly named protein complex named Dicer that chops it into short pieces, and then with another protein group called RISC that discards one of the two RNA strands. What’s left is a RISC-docked, single-stranded RNA looking for a match. In a kind of molecular ambush, the RISC complex binds and destroys the complementary messenger RNA that had been earmarked for protein production, silencing the gene.
This ability to dramatically reduce an individual protein inside of cells makes RNAi a valuable research tool. What was once a laborious process is now as easy as sneaking an RNA molecule into the cell with a sequence that matches the RNA a researcher wants destroyed. The technique can also be expanded to identify all the genes involved in a particular cellular event. The researcher can use an RNAi library to individually disrupt each gene from making its protein, then look for the ones that interfere with the event in question.
Fire was born at Stanford Hospital and raised in Sunnyvale, Calif. He received bachelor’s in mathematics in 1978 from UC-Berkeley. At the age of 19 he went to the Massachusetts Institute of Technology where he earned his PhD in biology in 1983. Fire was later a Helen Hay Whitney Postdoctoral Fellow in Cambridge, England, where he worked at an MRC Laboratory of Molecular Biology group headed by Nobel laureate Sydney Brenner. Between 1986 and 2003, Fire was a staff member of the Carnegie Institution of Washington’s Department of Embryology. The initial work on double stranded RNA as a trigger of gene silencing was published while Fire and his group were at the Carnegie Labs. Fire was an adjunct professor in the Department of Biology at Johns Hopkins University starting in 1989 and joined the Stanford faculty in 2003. Throughout his career, all of the major work in Fire’s lab has been supported by research grants from the US National Institutes of Health.
He is a member of the National Academy of Sciences and of the American Academy of Sciences. He also serves on the Board of Scientific Counselors and the National Center for Biotechnology, National Institutes of Health.
“Professor Fire's contributions to his field have been of enormous importance and the recognition by the Nobel committee is a remarkable achievement at this early point in his career,” said John L. Hennessy, president of Stanford University. “The RNA research of Professors Fire and Mello represents the very best of the collaborative nature of university scholarship. The fact that this basic discovery is already impacting the development of new therapies is a wonderful reminder of the importance of fundamental research. Stanford is indeed lucky to have a scientist of Professor Fire's caliber on its faculty. I offer him warm congratulations on behalf of the Stanford community.”
None of this has gone to Fire’s head. “I am still the same person,” he said on Swedish radio. “My goals are still fairly simple goals of research and science and teaching and family and I don't expect that to change.”
About Stanford Medicine
Stanford Medicine is an integrated academic health system comprising the Stanford School of Medicine and adult and pediatric health care delivery systems. Together, they harness the full potential of biomedicine through collaborative research, education and clinical care for patients. For more information, please visit med.stanford.edu.