July 31, 2012 - By Erin Digitale
BY ERIN DIGITALE

John Oghalai
Doctors should reconsider the common practice of avoiding the use of cochlear implants in deaf children with developmental delays, according to a new study from the Stanford University School of Medicine and Lucile Packard Children’s Hospital.
While cochlear implants are now routinely given to deaf children as young as 1 year old, physicians frequently opt not to use these devices — sometimes referred to as “bionic ears” — in babies with developmental delays that are indicators of probable mental retardation later in life. But the new findings suggest that the implants could substantially benefit these children’s intellectual development, even if their cognitive problems make it unlikely that they will ever learn to speak.
“If we can fix this deficit, it might help developmentally delayed children in other ways outside of hearing,” said John Oghalai, MD, the lead author of the study, which appears in the August issue of Otology & Neurotology. Oghalai is an associate professor of otolaryngology at Stanford and the director of the Packard Children’s Hearing Center.
The combination of deafness and developmental delay has become more common as increasing numbers of children survive extremely premature birth; complications of prematurity often include both cognitive delays and deafness.
But providing cochlear implants for developmentally delayed children has often taken a back seat to their other medical issues, Oghalai said. These children may be grappling with congenital heart defects, an inability to eat by mouth or serious breathing problems, for example, and doctors may not want to complicate their treatment with yet another procedure.
Physicians also have been wary of whether the implants would benefit the children. Cochlear implants send electronic signals from a microphone on the outside of the head directly to the auditory nerve, but the result is different from normal human hearing, and children typically require intensive speech and auditory therapy to learn to use and benefit from the implants.
For the study, Oghalai and his colleagues assessed use of cochlear implants in children who were cognitively normal and those who were developmentally delayed, a term used to characterize young children who may later meet criteria for mental retardation. The researchers reviewed records of 60 developmentally delayed and 144 cognitively normal children who received the implants, and found that the cognitively normal children got cochlear implants earlier in life than the developmentally delayed children. The former, on average, received them at 16 months of age vs. 25 months of age on average for the latter.
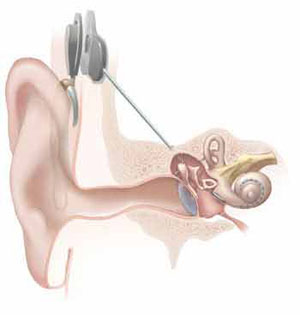
Illustration of a cochlear implant.
The researchers determined that later implantation had detrimental results. Not only did the delayed children start with lower intelligence, they also had slower intellectual development, perhaps because they spent more time unable to hear, the researchers reported. When the scientists statistically adjusted for the delay in implantation, the difference in rates of development disappeared, suggesting that lack of hearing plays a role in causing developmentally delayed children to fall further behind their peers.
“There is synergism between different sensory inputs,” Oghalai said. “And some of these kids are missing more than just hearing; they’re often having trouble with vision or touch as well. If you can fix one of the sensory problems, it might help to mitigate the effects of the other disabilities.”
Because standard scales for measuring intelligence were not appropriate for the young children being studied, part of the research team’s work was the development and validation of new methods for scoring the intelligence of these children. (For instance, some intelligence scales assess motor skills, which may be impaired if a child has physical disabilities unrelated to his or her intelligence level.)
Some deaf adults oppose using cochlear implants in young deaf children because they believe the implants will prevent the children from fully participating in deaf culture. But Oghalai said the debate is less relevant to developmentally delayed children who may not be able to be part of deaf — or mainstream — culture unless they can overcome the limitations of their other disabilities.
One drawback of the current study is that the developmentally delayed children who received implants were chosen on an ad-hoc basis. The next step in the research is a more systematic evaluation of whether cochlear implants benefit a large cross-section of developmentally delayed deaf children. A prospective trial funded by the National Institutes of Health to address this question is now under way at Packard Children’s, Oghalai said.
“The issue is to catch these children early when they’re in the neonatal intensive care unit, identify their hearing loss and be aggressive in following and treating them," Oghalai said. Instead of asking families to return for a hearing evaluation after their child’s other health problems have stabilized, which could take several months, infants with hearing loss are being given hearing aids by 6 to 8 weeks of age, then followed regularly in the audiology clinic with the goal of ensuring that they receive cochlear implants by 12 months of age, the minimum age approved by the FDA for use of the devices.
Managing families’ expectations of the power of the cochlear implants is also important, Oghalai noted. Cochlear implants will not make a developmentally delayed child cognitively normal, he said. “But if the implants can improve the children’s quality of life, make them happier and prevent them from falling further behind their peers, they may be a very worthwhile endeavor,” he concluded.
Oghalai’s collaborators include Barbara Bentley, PsyD, clinical psychologist in developmental and behavioral pediatrics; Homer Abaya, social science research assistant in otolaryngology; and Jody Winzelberg, AuD, chief of audiology and director of rehabilitation services at Packard Children’s.
The study was supported by the NIH. Information about Stanford’s Department of Otolaryngology-Head and Neck Surgery, which also supported the research, is available http://med.stanford.edu/ohns/.
About Stanford Medicine
Stanford Medicine is an integrated academic health system comprising the Stanford School of Medicine and adult and pediatric health care delivery systems. Together, they harness the full potential of biomedicine through collaborative research, education and clinical care for patients. For more information, please visit med.stanford.edu.